Removal Of Fluoride From Water By Iron-Oxide-Coated Sand
| 论文类型 | 技术与工程 | 发表日期 | 2001-09-01 |
| 来源 | 中国水网 | ||
| 作者 | 高乃云,Dimin,Xu,Jinchu | ||
| 关键词 | I ron-oxide-coated sand fluoride filtration adsorption isotherm | ||
| 摘要 | I ron-oxide-coated sand was compared with q uartz sand as a filter medium for removing fluoride from water solutions . The effect of the coated sand was confirmed to be noticeable , with the removal being able to reach a level of more than 90 percent . Th | ||
出 自: ASIAN WATERQUAL 2001 ― First IWA Asia-Pacific Regional Conference(Proceedings I Oral Presentation), Fukuoka, Japan; September 12-15,2001, p203-208.
发表时间: 2001-9
高乃云*, Dimin Xu*, Jinchu Fan*, Xushi Yan*, Akira Yuasa** and Fusheng Li***
* State Key Laboratory of Pollution Control and Resources Reuse, Tongji University, Shanghai, China ** Center for River Basin Environment Research, Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan *** Department of Civil Engineering, Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan
ABSTRACT
I ron-oxide-coated sand was compared with q uartz sand as a filter medium for removing fluoride from water solutions . The effect of the coated sand was confirmed to be noticeable , with the removal being able to reach a level of more than 90 percent . The removal efficiency was specially high at low er pH value s . For the quartz sand prior to the surface modification, h owever, the fluoride was not removed at all.
KEYWORDS
I ron-oxide-coated sand; fluoride; filtration ; adsorption ; isotherm
INTRODUCTION
Fluorine is an element and exists widely in the nature. Some local bone -related diseases are reported to be caused by chronic fluorosis, which is partially, if not totally, attributed to drinking water and foods polluted by fluoride . There are about 260 million people liv ing in high-concentration-fluoride bearing area s in China and their health has been gravely worried by possible fluorosis as statistical results collected from the northern part of China have clearly demonstrated a close relation between the removal of the fluoride from drinking water sources and the health conditions of people living there. A ccording to the World Health Organization , about 45-60 million people in China are drinking waters produced from fluoride-polluted water sources. For such reasons, the removal of fluoride-related pollutants through the drinking water processes has been considered imperative and many researchers have hence focused their work on this field. Rapid sand filtration is the most important process in drinking water production. However, its effect in removing the fluoride is reported to be very limited. The poor performance is likely to be contributed to the smaller surface areas and the negatively charged surface characteristics of the sand media used. Accordingly, the major objective of this research is to evaluate the effect of a surface-modified-sand medium prepared by coating quartz sand with iron-oxides in removing the fluoride from water solutions.
EXPERIMENTS
Preparation of iron-oxide-coated sand (IOCS) and its surface characteristics
Graded quartz sand was used to prepare both the conventional and the i ron-oxide-coated media. S and particles with sizes between 0.5 and 1.2mm were selected after being wash ed and dr ied . Coating was conducted by immersing 200g of the sand particles in a FeCl 3 solution controlled under a high temperature of 550 ° C for 3 hours. The coated particles were then mixed with 100ml of 2.5 - M FeCl 3 solution. The mixture was heated at 110 ℃ for 10 hours , and was then cooled to room temperature. T he coating was quite moist at beginning and reached stable after repeating the heating-cooling procedure for 3 times. The iron-oxide-coated sand (IOCS) particles were washed with distilled water to remove fines, dried , and finally stored until use.
The specific surface areas of the quartz sand and IOCS measured by the BET method were summarized in Table 1 . As show n, by coating the sand with iron-oxide, its surface area was greatly increased , with the specific surface area of the IOCS being approximately thirteen times as large as that of the original quartz sand. The enlarged surface area was mainly contributed to the accumulation of iron-oxide on the sand surface, as the analytical results from the X-ray diffraction analysis carried out at the Test Center of Fudan University clearly indicated that hematite ( α -Fe 2 O 3 ) was the major compound distributed on the surface of IOCS . The wave peaks of IOCS from the X-ray diffraction spectrum coincided with the peaks of card no.33-0664 in the Collection of Matter Standard Data stored in this University.
E xperimental apparatus and methods
Filtration experiments were conducted using two columns packed with IOCS and quartz sand with a depth of 40 cm, respectively. Sodium fluoride (NaF) was used to prepare the fluoride solutions for experiments. The solution was pumped into the columns in a flow rate of 4.08 m/hr and the effluent from the filters was sampled for quality analysis. To compare the removal efficiency of IOCS and the quartz sand, the two columns were operated in parallel.
RESULTS AND DISCUSSION
Effect of fluoride removal using IOCS and quartz sand
The removal history of fluoride by IOCS-packed filter is shown in Fig. 2. The results shown were obtained with respective to the following filtration condition: the influent concentration of 4.82 mg/L, the filtration rate of 4.08 m/h, the pH level of 3-5 and the operation time of 25 hours. At the very beginning of the filter run, the fluoride removal reached a higher level of more than 90% and such a higher removal tendency maintained unchanged for approximately 21 hours until a rapid decrease in the removal occurred at the final stage. Higher removals of the fluoride by the IOCS were confirmed to be associated with low pH levels although a marked difference in the fluoride removal was not observed over the pH range of 2.8 to 4.7 as shown in Fig. 3. The reason behind the pH dependency is to be discussed later .
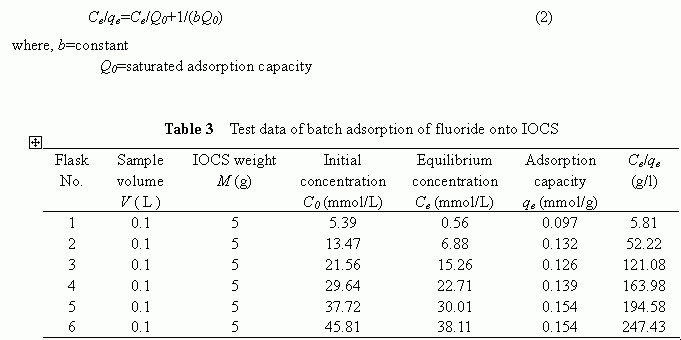
Compared to the IOCS, the quartz sand itself was totally ineffective in removing the fluoride from water solutions. The filtration results corresponded to a filter run time of 8 hours over the pH range of 6.6 to 7.1, as summarized in Table 2, confirmed this fact. It is conceivable that the specific surface characteristics of the quartz sand, for example, the negatively charged surface potential and the smaller surface area as compared to the IOCS, were the reasons responsible for its ineffectiveness in removing the fluoride.
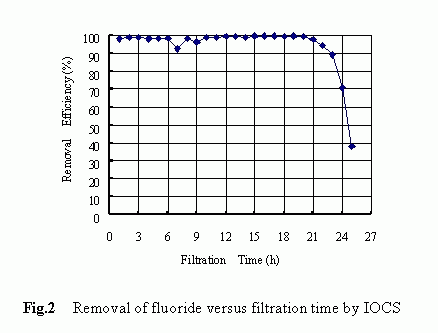
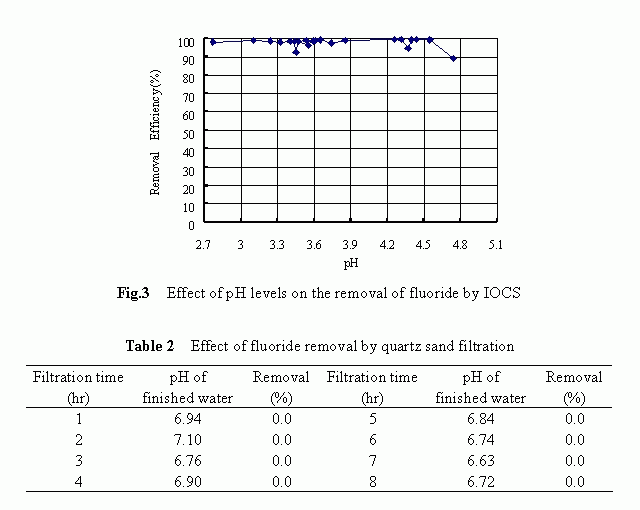
Above-mentioned results clearly demonstrated that the quartz sand itself was ineffective at all in removing the fluoride and that by modifying the medium surface with iron-oxides, a higher removal of fluoride could be achieved .
Adsorption isotherm of fluoride onto IOCS
To measure the adsorption isotherm of the fluoride onto the modified IOCS, the bottle-point method was employed to conduct a batch test for varying concentrations of the fluoride solutions under identical adsorbent dosages. The solutions with initial fluoride concentrations of 102.4 , 255.9 , 4 09.5 , 563.1 , 716.6 , and 870.2 mg/l were added respectively into six Erlenmeyer flasks along with 5 g of the IOCS . The ionic strength was adjusted with the addition of 10ml of 0.1 - M sodium chloride ( NaCl ) to prevent its likely effect on the adsorption capacities. After shaking t he f lasks at 100 rpm for 24 hours under a temperature of 28 ℃ , the supernatants were sampled and the liquid phase fluoride concentrations were measured. The adsorption capacities were calculated using the following mass balance equation.
qe=(Co-Ce)/(M/V) (1)

The equilibrium concentrations and the calculated adsorption capacities are summarized in Table 3. As
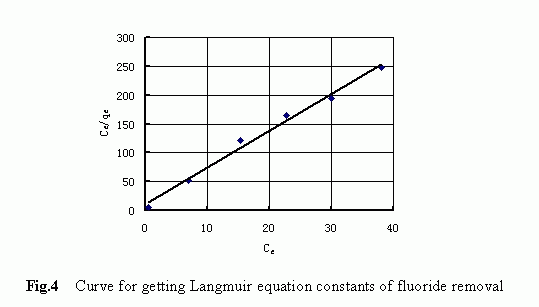
shown, the adsorption capacity increased as the equilibrium increased. The observed data were further analyzed with single adsorption isotherm models, i.e., the Freundlich isotherm model and the Langmuir isotherm model, to determine which model could better describe the adsorption equilibrium of the fluoride onto the IOCS. Analytical results indicated that, although both these models were good in describing the batch adsorption process, the Langmuir isotherm model as given below was considered more suitable as the difference noticed in the related correlation coefficients (R 2 =0.916 for the Freundlich model, R 2 =0.99 for the Langmuir model) suggested.
The adsorption isotherm plotted in terms of the Langmuir model is displayed in Fig. 4. The marks represent the observed data and the solid line represents the model fit. The good agreement between the model fit and the observed data confirmed that the Langmuir equation could be used to express the fluoride adsorption process and that t he adsorption of fluoride from water by the IOCS was a kind of single-layer adsorption process. The adsorption parameters related to the Langmuir model were determined as follows: b=0.584; Q 0 =0.158 (mmol/g).
Mechanism of fluoride removal
Langmuir model was derived on the basis of both statistical thermodynamic and kinetic considerations. The model was based on the assumption of localized adsorption, with no interactions between the adsorbed molecules, with each adsorption site distributed on the adsorbent surface being occupied by no more than one molecule, and with all adsorption sites possessing equal adsorption energies. Furthermore, due to adsorptive interactions, adsorption was assumed to be immobile, i.e., transmigration of the adsorbate in the plane of the surface was precluded. The capacity of adsorption could reach the largest value when the adsorption sites were fully occupied to form a single-layer coverage of the adsorbate. When sodium fluoride (NaF) was dissolved in the distilled water , it dissociated as follows:
![]()
论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 paper@h2o-china.com ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








