The Investigation of Chlorine Dioxide Purity Determined by M
| 论文类型 | 技术与工程 | 发表日期 | 2005-12-01 |
| 作者 | Laishun,Shi | ||
| 关键词 | Chlorine dioxide Generator Purity Sodium chlorate Hydrochloric acid | ||
| 摘要 | The UV and IR spectra of mixture of chlorine dioxide and chlorine, produced from chemical chlorine dioxide generator, were measured. It is favorable to raise the purity of ClO2 by the reaction of sodium chlorate with hydrochloric acid if the reaction temp | ||
The Investigation of Chlorine Dioxide Purity Determined by Modified Iodimetric Method
Laishun Shi
(College of Chemistry and Chemical Engineering, South District,
Shandong University, Jinan 250061,P. R. China)
Abstract The UV and IR spectra of mixture of chlorine dioxide and chlorine, produced from chemical chlorine dioxide generator, were measured. It is favorable to raise the purity of ClO2 by the reaction of sodium chlorate with hydrochloric acid if the reaction temperature was elevated. The purity of ClO2 was also raised greathly by purification of urea.
Keywords Chlorine dioxide, Generator, Purity, Sodium chlorate, Hydrochloric acid
1. Introduction
As a strong oxidant, there is not chlorine substitute carcinogenic substances, in the production of water treated by chlorine dioxide. As a disinfectant, chlorine dioxide belongs to high efficiency, low toxicity and able to kill various bacterium and versus. Therefore, chlorine dioxide has applied extensively in the field of water treatment. According to literature, the angle of the two double bond of C=Cl is 117.7±1.7°in the molecular of chlorine dioxide. The distance of the two oxygen atoms with the chlorine atom is equal. That is, D=1.784±0.01. The characteristic infrared spectrum absorption frequency is 945 cm-1, 445cm-1 and 1108cm-1. The ultraviolet absorption wavelength of chlorine dioxide in carbon tetrachloride is λmax=375nm and 355nm. It has a weak absorption peak at 263nm. Author has investigated the purity of chlorine dioxide in the chlorine dioxide water disinfectant generator by tyrosine colorimetric method in the previous papers[1-3]. It has found that there is a stronger absorption peak at 306nm, except the color-reaction absorption peak at 490nm. This paper studies the influence of reaction temperature and purified method to the purity of chlorine dioxide by using the principal of different reaction of chlorine dioxide and chlorine with iodide under different pH value.
2. Experimental
2.1 Reagent and Instrument
0.01mol/L standard solution of sodium thiosulphate, KBr, hydrochloric acid, 1% starch indicator, and KI are analytical purity reagent. Urea is chemical purity. Sodium chlorate is industrial product. Buffer solution of pH=7 is obtained by weighing 25.4g anhydrous KH2PO4 and 86g Na2HPO4.12H2O, and dissolved in 1000ml distilled water.
UV spectrum was obtained by using U-2000 double light beam spectrophotometer (Hitache) . IR spectrum was obtained by Perkin-Elmer 983 model infrared spectrophotometer. Chlorine dioxide generator was made by myself.
2.2Determination of Chlorine Dioxide Spectrum
The sample of chlorine dioxide is obtained by dissolving chlorine dioxide and chlorine in carbon tetrachloride. The UV spectrum was measured on the U-2000 double light beam spectrophotometer by using carbon tetrachloride as the reference solution. The IR spectrum of chlorine dioxide and chlorine in carbon tetrachloride was measured on the Perkin-Elmer 983 model infrared spectrophotometer.
2.3Determination of Chlorine Dioxide Purity
Preparation of chlorine Dioxide Samples: The raw materials of the generator is sodium chlorate and hydrochloric acid. No purified sample of chlorine dioxide and chlorine is obtained by absorbing chlorine dioxide and chlorine gases produced from the generator with distilled water. Purified sample of chlorine dioxide and chlorine is obtained by absorbing chlorine dioxide and chlorine gases with distilled water, which is first produced by the generator and then purified by urea aqueous solution of certain concentration for 2min.
Analysis method: To 250ml iodometric flask, 10ml buffer solution of pH7, accurate 20ml chlorine dioxide and chlorine aqueous solution, and 0.5 gram potassium iodide was added, then put at dark place for 5min. The solution was titrated to pale yellow by using sodium thiosulphate standard solution, added 1ml starch indicator, titrated again to blue color disappeared, The volume consumption (V1) of sodium thiosulphate standard solution was recorded. 5ml 6mol/L hydrochloric acid was added to the above solution. The solution was changed to deep blue, and them put at dark place for 3min. The solution was titrated to blue color disappeared. The volume consumption (V2) of sodium thiosulphate standard solution was recorded.
3. Results and Discussion
3.1 The Spectra of chlorine Dioxide
Figure 1 represents the UV spectrum of chlorine dioxide and chlorine in carbon tetrachloride solution. There is a strong absorption peak at 190~270nm, which covers the weak absorption peak at 263nm. Also, there is a wide band multiple peak at 320~420nm. The highest peak value is 355nm. Figure 2 represents the IR spectrum of chlorine dioxide and chlorine in carbon tetrachloride solution. The peaks of 1253、1218、1006、979、777、628cm-1 are characteristic absorption frequency of carbon tetrachloride. The frequency of 750~850 cm-1 is C-Cl stretch vibration. The frequency of 1094 cm-1 and 938 cm-1 is characteristic absorption peaks of chlorine dioxide.
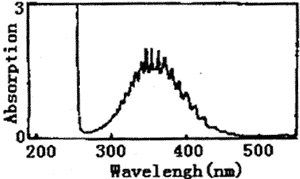
Fig.1 The UV spectrum of chlorine dioxide and chlorine in carbon tetrachloride solution
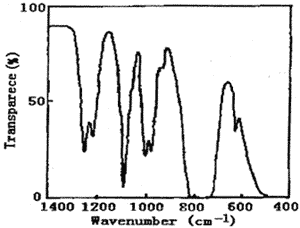
Fig.2 The IR spectrum of chlorine dioxide and chlorine in carbon tetrachloride
2.1. Purity Analysis of /chlorine Dioxide
When measuring the purity of chlorine dioxide, the principle is based on the follow reaction.
When pH=7, Cl2 + 2I- = I2 + 2Cl-
2ClO2 + 2I- = I2 + 2ClO2-
When pH=2, 2ClO2 + 10I- + 8H+ = 5I2 + 2Cl- +4H2O
ClO2- + 4I- + 4H+ = 2I2 + Cl- + 2H2O
When pH is 7, KI can change Cl2 to Cl-, ClO2 to ClO2-. When pH is 2, ClO2- can further reacted with KI to produce Cl-. According to the volume consumption of sodium thiosuphate standard solution at different pH value, the purity of chlorine dioxide in chlorine dioxide in chlorine dioxide and chlorine mixed gases can be calculated.
V2
ClO2%(based on effective chlorine mass fraction)= (5/4)*------------- *100%
V1+V2
In the formula, V1 (ml) represents the volume consumption of sodium thiosulphate standard solution at pH=7. V2 (ml) represents the volume consumption of sodium thiosulphate standard solution at pH=2.
Table 1 listed the data of chlorine dioxide purity before and after purification of urea for the chlorine dioxide and chlorine gases by the reaction of sodium chlorate with hydrochloric acid at different temperatures. Based on the table, we can get the conclusion that the purity of chlorine dioxide is increasing along with the increase of reaction temperature for the no purified sample. That is to say, temperature increasing favors the production of chlorine dioxide. The reaction is,
NaClO3 + 2HCl == ClO2 + 1/2Cl2 + NaCl + H2O (1)
Theoretically, the chlorine dioxide purity of the above reaction is %. The actual results are below than the theoretical. It indicates that there is a parallel reaction at the same time, which favors the chlorine formation. The reaction is [5],
NaClO3 + 6HCl == 3Cl2 + NaCl + 3H2O (2)
Temperature increasing favors the reaction of (1), disfavors the reaction of (2).
Based on the data of urea purification at same urea concentration, we can also get the same conclusion that the purity of chlorine dioxide is increasing along with the increase of reaction temperature. By comparing the data of before and after purification at the same reaction temperature, the purity of chlorine dioxide is increasing along with the increase of urea concentration. This is because chlorine can reacts with organic and inorganic substance containing ammonia, e.g., ammonia, urea, to produce chloroammonia, dichloroammonia, and nitrogen[6]. Chlorine dioxide cannot react with ammonia, therefore urea can get the purpose of purification of chlorine dioxide.
Table1 The purity of chlorine dioxide
4. Comclusions
(1) The UV and IR spectra of chlorine dioxide and chlorine produced from chlorine dioxide generator coincides with the literatures.
(2) The temperature increasing of the sodium chlorate react with hydrochloric acid favors the formation of chlorine dioxide.
(3) The chlorine dioxide purity can be greatly increased by the purification of urea solution for the chlorine dioxide generator.
References
1. Laishun shi, et al. Colorimetric and Absorption Intensity Method for the Determination of Chlorine Dioxide in Water. J. Chinese Disinfection, 1996, 13(4):240-241
2. Laishun shi, et al. Experimental Observation of Killing Microorganism by Using Chlorine Dioxide. J. Chinese Disinfection, 1996,13(1):30-33
3. Laishun Shi, et al. Colorimetric Method for the Determination of Chlorine Dioxide in Water. J. Here Teachers College, 1998, 20(4):39-41
4. Aieta E M, Roberts P V, and Hernandez M. Determination of Chlorine Dioxide, Chlorine, Chlorite, and Chlorate in Water. J. AWWA, 1984,76(1):64-70
5. Tenney J, Shoaei M, Obijeski T, et al. Experimental Investigation of a Continuous Chlorine Dioxide Reactor. Ind. Eng. Chem. Res., 1990,29:912-916
6. Chaoren Xie, Laishun Shi. The Technology of Swimming Pool Water Purification. Jinan: Jinan Press (1993)
close
www.h2o-china.com论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 paper@h2o-china.com ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








