Apparent Molecular Weight Distribution of NOM in Macau Water
| 论文类型 | 基础研究 | 发表日期 | 2005-10-01 |
| 来源 | Macau Environment and City Development 2002 | ||
| 作者 | Wong,Hou,Lou,Wei,Man | ||
| 关键词 | AMW THM | ||
| 摘要 | The performance of the Macau water treatment plants in the removing various apparent molecular weight (AMW) fractions of naturally occurring aquatic organic matter and humic substances is described. Ultrafiltration method is applied in this research for s | ||
Macau Environment and City Development 2002
Apparent Molecular Weight Distribution of NOM in Macau Water and its Relation with the THM Formation
Wong Hou, Lou Wei Man, The Macau Water Supply Co. Ltd.
Li Shuang, Zhang Xiaojian, Tsinghua University
K. M. Mok, Wang Zhishi, University of Macau
(Faculty of Science and Technology, University of Macau)
(Laboratory and Research Center, Macau Water Supply Company)
Abstract: The performance of the Macau water treatment plants in the removing various apparent molecular weight (AMW) fractions of naturally occurring aquatic organic matter and humic substances is described. Ultrafiltration method is applied in this research for separation of the NOM in apparent molecular weight base. It is proved that Macau raw water has a very low TOC value and the apparent molecular weight lower than 3000 is the dominate fraction. As a general rule, THM reactivity (mg THM / mg C ) increased with AMW.
Keywords: AMW, THM.
1. General Introduction
Surface water and, to a lesser extent, groundwaters contain naturally occurring dissolved organic matter (NOM), including humic substances. Thurman et al. indicated that the aquatic humic substances generally account for about 30% - 50% of the dissolved organic carbon in natural water sources. Aquatic humic substances are important from a water treatment perspective for several reasons, including their role as precursors in the formation of chlorination byproducts such as trihalomethanes (THMs) and other organo-Cl compounds. In addition, humic substances may be effective in the concentration and transport of inorganic and organic pollutants. Humic substances also complete with regulated chemicals for adsorption sites on activated carbon and can cause fouling of ion exchange and reverse osmosis membranes. Because of the problems associated with humic substances, their removal during water treatment must be optimized. Coagulation provides substantial removal of humic substances. The variability of THM precursor removal by water treatment plants can be due to both operation conditions as well as the specific characteristics of the humic material itself. Researchers (Oliver, B. et al. 1980) have shown that the THM yield or reactivity of humic substances from a given source as expressed in mg THM/ mg C, varies as a function of molecular weight (AMW). Molecular weight for aquatic humic substances has been reported to range from 500 to 100000 Daltons. Thurman et al. (1982) suggested that this wide variation maybe due to the source of the humic substances, the analytical methodology used, and the aggregation of humic substances.
Since the city does not have large plenty of fresh water resource, source water (raw water) is taken from the Modaomen which is one of the eight widest estuaries of the Pearl River System. It is a main outlet of West River to the South China Sea. The runoff of West River accounts for 70% of the total runoff of Pearl River while that of Modaomen accounts for 28.67% of that of West River. The runoff of Modaomen is the biggest one in all the eight estuaries. The main water treatment plant located at Ilha Verde in Macau (maximum daily water supply 175000 m3) applied the conventional water treatment technique, which includes pre-chlorination, coagulation, flocculation, sedimentation and filtration then final disinfection by chorine. Raw water is mainly supplied by the Mainland China. According to different water treatment technique of the plant, the efficiencies of each of it will be evaluated by comparing the AMW of the NOM before treatment and after the treatment, meanwhile, the raw water characteristics is analyzed. The water sample we used to perform the ultrafiltration UF sample is the main water treatment plant in Ilha Verde, water samples during each treatment step was analyzed.
2. Related Work
2.1. Ultrafiltration
Ultrafiltration involves the separation of molecules from a liquid phase according to molecular size. Migration of molecules through membranes is generally accomplished by a combination of advective flow and molecular diffusion. The flux of a solute is
directly related to the area of membrane, concentration gradient, and molecular diffusion, but is inversely related to temperature. (Brock, T.D. 1983)
One main problem of ultrafiltration is called concentration polarization, the deposition of macromolecules on the membrane surface which results in a gel layer that becomes the dominant resistance to flow. This will appeared as the slow downed flux rate. Thus, an important requirement when using a membrane for AMW determinations is to ascertain when gel-layer diffusion, indicating that the membrane should be replaced or cleaned. As recommended by Macko et al and Brock, vigorous mixing of the feed solution is required in order to minimize the thickness of the membrane surface. Another suggestion is to keep the concentration of the solute as low as possible. Another problem arise is known as the Donnan effect, in which one of the solutes is unable to permeate the membrane. The phenomenon may lead to an unequal distribution of ions across the membrane, and, because electrical neutrality must be maintained, the membrane develops a potential that trends to impede the diffusion of any more ionic species that are similar in charge to that of the membrane. It is later suggested by Macke that the solution‘s pH and ionic strength should be held constant for uniform result.
The reason for selecting ultrafiltration is not only because of the easy solution of the induced technique problems and availability of equipment, but also due to another important requirement, the fractionated water sample according to different AMW after the ultrafiltration can be used to perform the THMFP test, this is main character of water sample of ultrafiltration which is totally cannot be satisfied by other techniques. The other methods can only provide the AMW distribution of analyzed water sample while cannot separate the different fraction of water sample accordingly. The advantage of ultrafiltration method attracts us to make this decision.
According to the literature, 75% of the THMs formed were derived from organics having a molecular weight of < 3000 Daltons. Moreover, 7% of the organics and 20% of the THMs were derived from compounds of <1000 Daltons molecular weight, in a related study, Veenstra and Schnoor (1980) found that the greatest THM yield per unit of organic carbon occurred in conjunction with molecules with an apparent molecular weight of <1000 Daltons. The molecular weight of aquatic humic substances generally ranges from approximately 500 - 10000 Daltons. The UF results for both raw and treated waters provided indicate relatively small differences between the THMFPs of the < 10000 Daltons fraction, the < 30000 Daltons fraction, and the overall humic content of the water (i. e., the 0.45mm filtrate). This result suggests the presence of little precursor material with an AMW of greater than 10000.
3. Experimental Method
3.1. Water Sample.
Four water samples was taken along the water treatment process, influent water ( raw water and source water ), coagulated water, filtrated water and treated water.
3.2. Molecular Weight Determination
The apparent Molecular weight of the NOM in the water is determined by the membrane ultrafiltration method. Before the ultrafiltration, raw water and the coagulated water is filtrated by the 0.45mm membrane ( Millpore, F47 mm. ) to remove the suspended solid. Apparent molecular weight distributions were obtained using a stirred cell (Amicon Inc, model 8400, effective volume 350ml) with membranes characterized by nominal AMW cutoffs of 1000, 3000, 10000, 100000 (YM1, YM3, YM10, YM100; Amicon Inc.). Sample aligquots were processed through the range of membranes in parallel, yielding a series of permeates, each containing all molecular weight fractions below the indicated
AMW cutoff, all permeates were characterized according to DOC, UV absorbance and THMFP.
3.3. Organic Carbon and THM Analysis
Each step of the laboratory experiment is following the company SOP which is certificate by the CNACL 201-99 (equal to ISO/IEC). The water samples were sampled by dark glass bottles which were baked to the temperature of 500oC. The permeates test for the DOC (Shimadzu Int, model number TOC-5000A.) and UV254 (Shimadzu Int, model number UV-2401) after the filtration. During the THMFP experiment, the permeates were contained in a 50ml dark glass bottle and the initial chlorine concentration is about 20ppm. Incubated for 3 days under the temperature of 25oC in darkness then adding Na2SO3 to stop the reaction. The final water sample is being used for the testing of final THM. All water sample is analyzed in the company laboratory. The THM concentration is analyzed by the HPGC, which is the product of Perkin-Elmer.
4. Results and Discussion
Besides the raw water, the other water samples taken practiced prechlorination. A summary of chemical addition and operating conditions of water samples are presented in Table 1.
Table 1 Results of Ultrafiltration.
4.1. The apparent molecular weight ( AWM ) distribution of DOC, SUVA and THMFP along water treatment process.
The result of the apparent molecular weight distribution of the DOC, SUVA and THMFP variance along the water treatment process is presented in the Figure1, Figure 2 and Figure 3. DOC concentration of the Macau raw water is about 1.5~2 ppm normally, without much big variance along a year, the UV254 value is about 2.5~3.5 m-1, accordingly, the SUVA value is always well below 3. According the statement of Edzald, this type of water has mainly the nonhumic material existed in the organic matter. The dissolved material is hydrophilic and comparatively less aromatic material existed in the small molecular weight organic materials. The coagulant has low effect on the remove of the DOC.
From Figure 1, the DOC remove efficiency is lesser than 20%. Especially for the DOC which molecular weight lowers than 1000 Daltons, the water treatment process hardly has any effects on that fraction of permeates. However, the organic matter which has the molecular weight lower than 3000 Daltons occupies about 80% of the total DOC concentration, almost 50% of the total DOC has the molecular weight lower than 1000. According to the literature, These observation obeys the opinion of Edzwald, for the nonhumic type water, low molecular weight organic dominate the DOC, and the conventional water treatment process has low effect on remove it.
The specific ultraviolet absorbance (SUVA) of a water which is defined as UV absorbance (measured in cm-1) time 100 divided by the dissolved organic carbon concentration (mg/L), has been found to be a good surrogate for the water‘s humic content (Edzwald and Van Benschoten 1990 ). In Figure 2, high SUVA value is observed at the low molecular weight range of raw water (IR), this may imply that at a lower molecular weight range, there are more conjugated double bonds available to react with chlorine to form DBPs. From the line of treated water (IT), the SUVA value has much lowed, this may due to the chlorination effect. Referring to the AMW lower than 3000 Daltons, the SUVA has been decreased a lot after the treatment, this evidence indicate the most of the humic material existed in low molecular weight fraction of raw water has been much oxidized by the chlorination to form DBPs.
The THMFP is defined as the formation potential of trihalomethane, which is a well-known disinfection by-product because of its high molar mass fraction. In Macau this species is much concerned in some specific situation ( i.e. salt water intrusion during dry season.). Figure 3 shows the distribution of the THMFP on the AMW base. There are three important observations. First, there is a very significant decrease of THM procures appears between the raw water (IR) and the coagulated water (IM), this provided the effect of the prechlorination and coagulation on removing the organic matter of molecular weight higher than 100000 Daltons. Unfortunately, the precursor with the AMW lower than 100000 Daltons did not share this benefit, it is clear to see for the precursors with molecular weight lower than 3000 Daltons, the concentration has not large change despite of the second chlorination effect of the treated water (IT). Second observation is the dominate AMW fraction of the precursors in treated water is the
molecular weight lower than 1000 Daltons, it occupies about 95% of the total precursors existed in treated water. Third observation is almost all the THM precursors is concentrated on the molecular weight around 1000 Daltons, this fraction is also the part which is most difficult to be remove by conventional treatment plant, has the dominate effect on the THM formation.
4.2. THM reactivity along water treatment process.
The THM reactivates or yields (expressed in terms of mg THM/mg C ) is shown in Figure 4. The average reactivity of the THM is around 1.2% ~ 0.8% for the raw water and 0.8% ~ 0.5% for the treated water. The reactivity of the THM is increasing along the
Decreasing of the AMW, both in raw water and treated water while the AMW lower than 1000 Daltons has almost (for the IT AMW<3000, the reactivity is also very high.) the highest reactivity. Coagulation has proved its effect on reducing the THM reactivity, both for the AMW > 100000 and lower than 1000 Daltons, the reactivity of THMs decreased according to coagulation (IM). These two same decreasing trends are caused by different reasons. For the part AMW>100000 Daltons, the decreasing of reactivity is induced by the removing of larger organic matter by coagulation and flocculation process. However the AMW < 1000 Daltons, the decreasing reactivity is induced by the first and second chlorination oxidized the most of the humic acid which concentrated in the low molecular weight fraction of organic matter.
Focusing on the treated waters, it can be seen that the aquatic organic matter remaining after treatment in each molecular weight range was generally less reactive in formation THMs in comparison to the untreated water. These results suggest that all of the various treatments preferentially remove the most reactive fraction of precursor present in each molecular weight range (Michael R. Collins, Gary L. Amy).
When comparing the figure of SUVA and THM formation reactivity (Figure 5), the trending of each corresponding curve is alike. Indicate the evidence that the SUVA is a good indicator for the trending of the reactivity of THMs in both raw and treated water.
5. Conclusion
Molecular weight for aquatic humic substances has been reported to range from 500 to 100000 Daltons from the literature. About 80% of the aquatic organic matter in Macau raw water has the AMW lower than 3000 Daltons, more advance, for the portion of AMW lower than 1000 Daltons it occupies about 50% of the total aquatic organic matter. The SUVA value of Macau water is always blow 3, from this two observation, we would conclude that Macau has a kind of low humic content source water and the aquatic organic matter existed in source water is dominated by low molecular weight. This factor has constrain the efficiency of coagulation so that is why coagulation has no significant efficiency on removing the organic matter in it (removing efficiency around 10%), for the organic matter of AMW lower than 1000 Daltons, the removing efficiency is almost 0. The humic material is concentrated on the fraction of AMW lower than 3000 Daltons (70%), the total removing efficiency is around 40%, both coagulation (12%) and filtration have effects on removing the humic materials. It is also expect that the first and second chlorination should also has efforts, however, there is no enough evidence to prove it. In Figure 3, the portion of AMW lower than 1000 Daltons dominate the THMFP generation (50%). Coagulation can remove a large portion of organic matter with AMW higher than 100000 Daltons. The reactivity of THM can be indicated by the factor of SUVA, it is clear to see that the SUVA value increasing along the decreasing of
The AMW of organic matter. After the treatment, both the reactivity and the SUVA value have been lowered. According to the statement of Michael R. Collins, all of the various
Treatments preferentially remove the most reactive fraction of precursor present in each molecular weight range. Despite of the fact that most reactive portion is existed in the organic matter with AMW lower than 1000, the reactivity of the each fraction is decreased along the treatment.
References
Amy G.L. and Collins M.R. 1987. Comparing Gel Permeation Chromatography and Ultrafiltration for the Molecular Weight Characterization of Aquatic Organic Matter. In Research and Technoloty.
Brock T.D. 1983. Membrane Filtration. In Sci. Tech. Inc.
Collins M.R. and Amy G.L. 1985. Removal of Organic Matter in Watr Treatment. In ASCE National Conference on Environmental Engineering.
Edzwald, J.K. and J.E. Van Benschoten. 1990. Aluminum Coagulation of Natural Organic Matter. In Proc. Fourth Int‘l Gothenbury Symposium on Chemical Treatment, Madrid, Spain.
Oliver B. and Visser S. 1980. Chloroform Production from the Chlorination of Aquatic Humic Material: The Effect of Molecular Weight, Environment, and Season. In Water Research, Vol.14.
Therman E. and Wershaw R.L. et al. 1982. Molecular Weight of Aquatic Humic Substances. In Organic Geochemistry, Vol. 4. P27.
Veenstra J.N. and Schnoor J.L. 1980. Seasonal Variations in Trihalomethane Levels in an Iowa River Water Supply. In Journal of AWWA, 72:10:583.
Figures
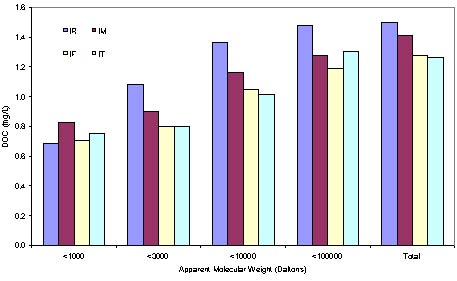
Figure 1. DOC variance along water treatment process.
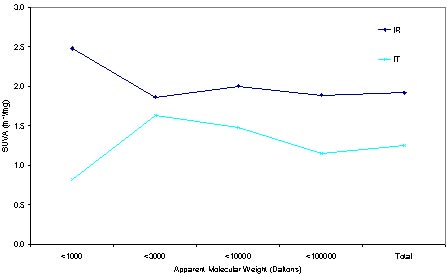
Figure 2 SUVA comparison of the influent water and treated water along treatment process.
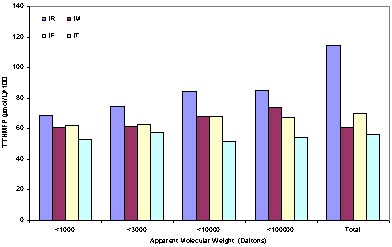
Figure 3 The THMFP variance along water treatment process.
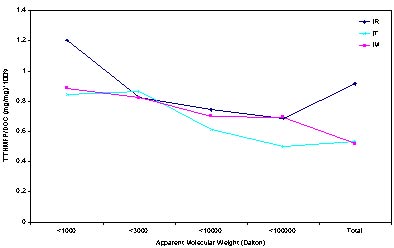
Figure 4 THM reactivity along water treatment process.
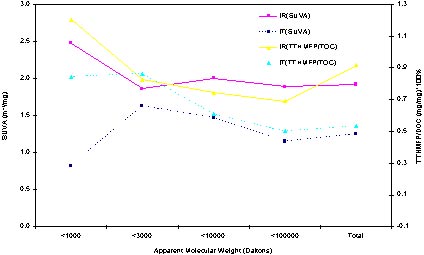
Figure 5 The comparison of SUVA and THM reactivity.
close
www.h2o-china.com论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 paper@h2o-china.com ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








